Development Pipeline
Tempest is developing a first-in-class oncology pipeline of oral therapeutics with broad potential for patients. The product candidates are designed to treat cancer by direct tumor killing, activating the immune system to kill tumor cells, or a combination of both mechanisms.
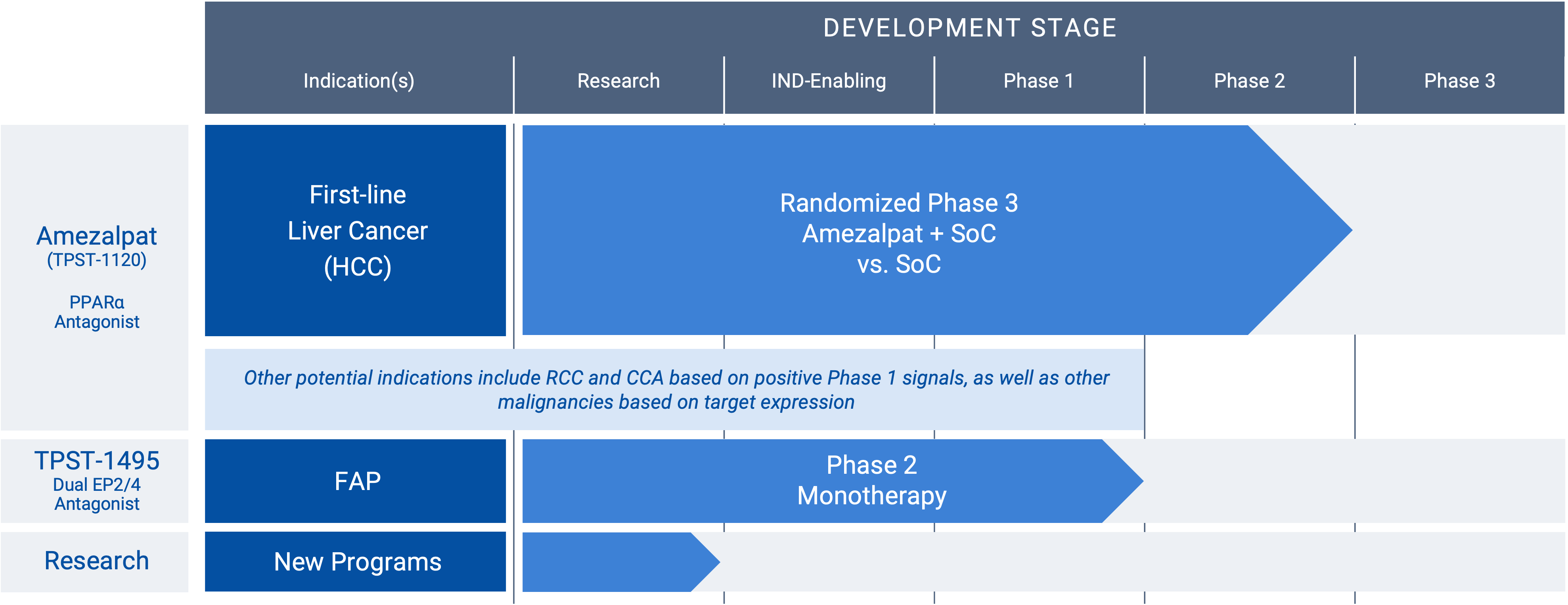
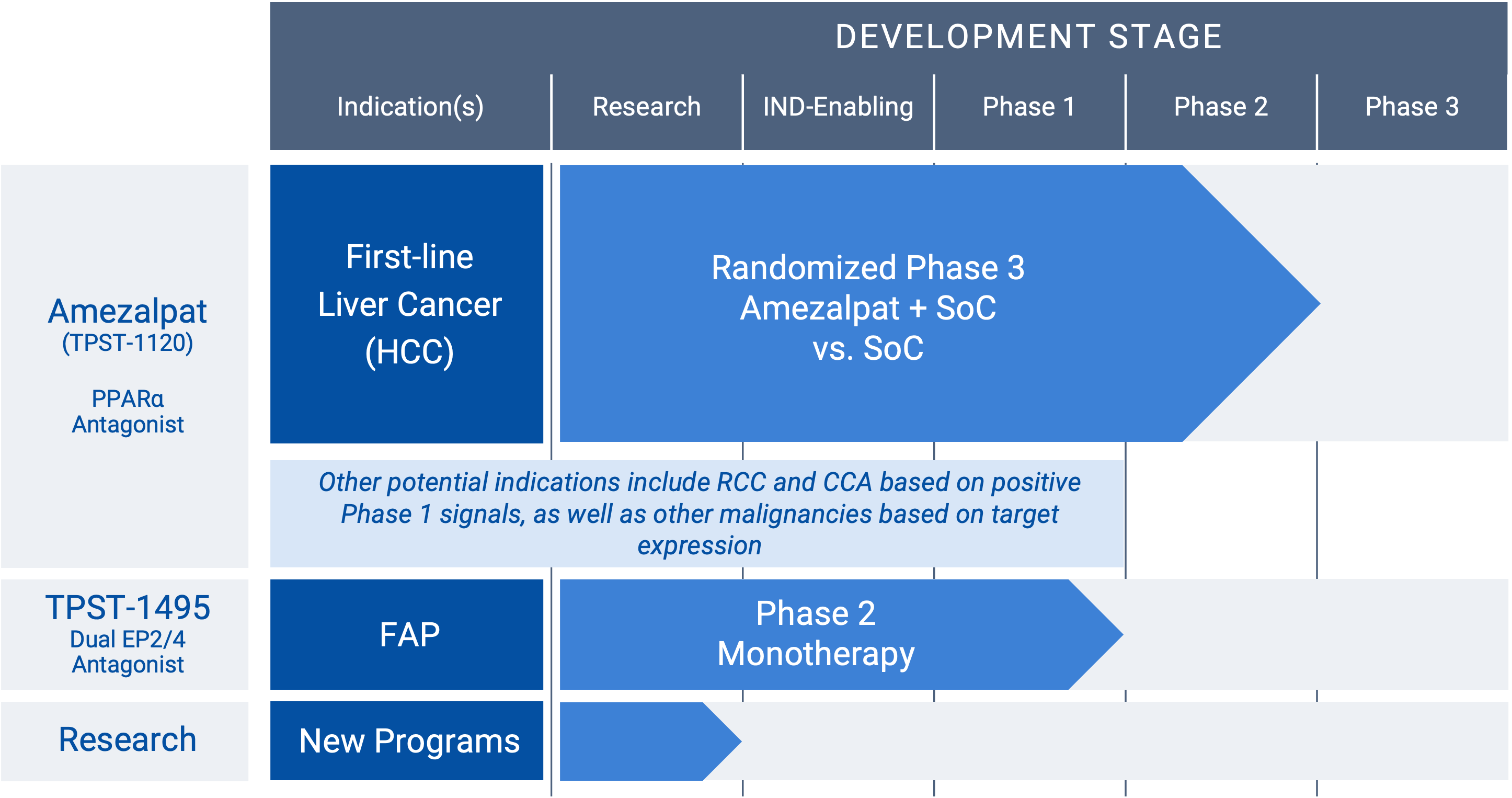
“RCC” renal cancer; “HCC” hepatocellular carcinoma; “CCA” cholangiocarcinoma; “FAP” familial adenomatous polyposis
First in class if approved by FDA. 1 Pursuant to a collaboration with Roche; TPST retains all product rights.
Programs
Amezalpat (TPST-1120) (PPARα antagonist)
Amezalpat (TPST-1120) is a first-in-class oral antagonist selective for peroxisome proliferator-activated receptor alpha (PPARα). PPARα is a transcription factor that regulates fatty acid oxidation (FAO) and inflammation and is over-expressed in many cancers and in immune cells. A PPARα deficiency in both tumor cells and the host shows reduced angiogenesis and a failure to support tumor growth. Amezalpat is a competitive antagonist of PPARα that can displace endogenous and synthetic agonists resulting in inhibition of FAO gene expression, a key metabolic adaptation of cancer and immunosuppressive cells. In addition, amezalpat may block the ability of PPARα to transrepress NF-kB resulting in reversal of immune suppression, establishing a rationale for amezalpat to fight cancer by dual mechanisms.
Hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), and cholangiocarcinoma (CCA) are three cancers that express high levels of PPARα and its multiple target genes. After presenting Phase 1 data in an oral presentation at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Tempest released positive data from a global randomized Phase 1b/2 study in combination with atezolizumab (TECENTRIQ®) and bevacizumab (Avastin®) in first-line patients with advanced HCC pursuant to a collaboration with F. Hoffmann-La Roche Ltd. The positive data showed amezalpat (TPST-1120) delivered a six-month improvement in median overall survival (OS) when combined with atezolizumab and bevacizumab, the standard of care in first-line HCC, when compared to standard of care alone. In addition, survival benefit from the addition of amezalpat was preserved in key sub-populations including PD-L1 negative disease and b-catenin mutated disease, which is consistent with amezalpat’s proposed mechanism of action to target both the tumor cells directly and the patient’s immune system. Tempest plans to advance amezalpat (TPST-1120) into a pivotal study in first-line HCC patients, as well as explore development beyond HCC given the signals observed in the Phase 1 study.
TPST-1495 (dual EP2/ep4 antagonist)
A first-in-class oral antagonist selective for two receptors in the prostaglandin (PGE2) pathway, EP2 and EP4. COX-2 expression and increased prostaglandins synthesis are hallmarks of malignant tumor progression. Prostaglandin E2 signals through four receptors (EP1-4). While EP1 and EP3 are antitumorigenic, EP2 and EP4 drive both tumor growth and immune suppression. The EP2 and EP4 receptor targets are highly expressed in diverse malignancies, including colorectal, lung, gastric and endometrial cancers. Redundant signaling through both EP2 and EP4 suggest developing a potent dual antagonist would have significant benefit for patients. TPST-1495 is a potential orally bioavailable first-in-class EP2/4 dual antagonist that has completed monotherapy dose escalation. The company plans to advance TPST-1495 into a Phase 2 study in patients with Familial Adenomatous Polyposis (FAP) in 2025 under the auspices of the Cancer Prevention Clinical Trials Network and funded by the National Cancer Institute (NCI) Division of Cancer Prevention, subject to final approval of NCI.
Research
Tempest Therapeutics is a biotechnology company focused on developing novel immunotherapies for the treatment of cancer. The company’s research interests lie not only in leveraging different approaches to target tumor cells directly, but also in harnessing the power of the immune system to target and eliminate cancer cells. The Tempest team is particularly interested in developing therapies that modulate the tumor microenvironment, enhance immune cell function, and overcome immune suppression within the tumor. It is our goal to develop innovative treatments that improve outcomes for cancer patients.
OUR EXPANDED ACCESS POLICY (EAP) TO INVESTIGATIONAL DRUGS
- Tempest understands that there are times when an individual is unable to participate in a clinical trial, and other treatment options have been exhausted. In those cases, the individual's physician may choose to request access to an investigational drug outside of a clinical trial via what is often termed “Expanded Access” in the United States.
- At this time, participation in a clinical trial is the only way to gain access to Tempest’s investigational therapies. As more clinical data on the safety and efficacy of these investigational therapies become available, Tempest may review and update this policy on Expanded Access.
