TREX-1 Inhibition
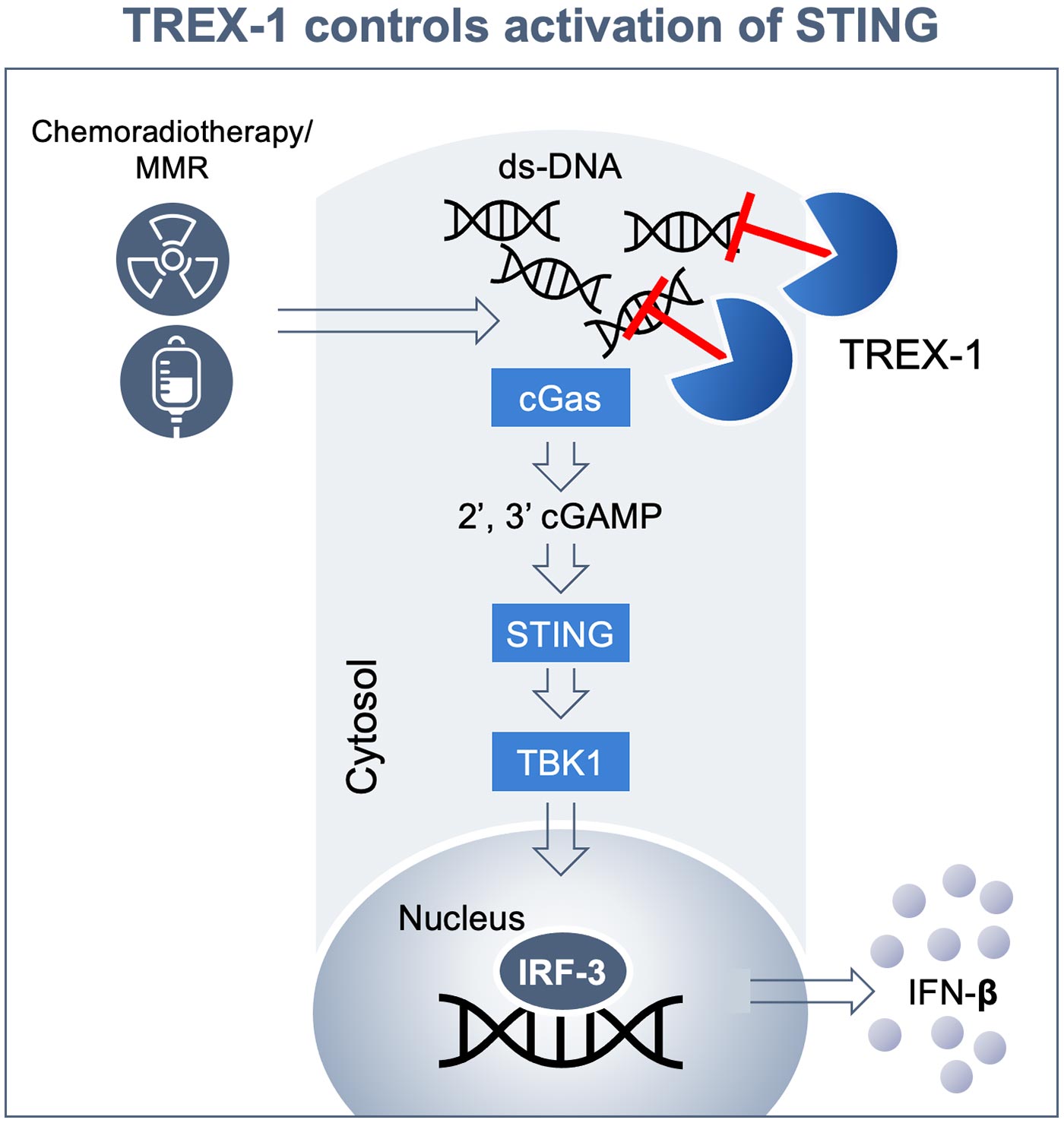
The exonuclease TREX-1 is the sentinel of cytosolic dsDNA sensing and dampens the activation of cGAS/STING to avoid immune recognition. TREX-1 is induced in response to DNA damage due to genetic instability of tumor cells and various therapeutic interventions including chemoradiotherapy or targeted therapies such as PARP inhibitors.
Inhibition of TREX-1 with a potent orally available inhibitor will preferentially localize activation of STING to the TME and prime T cells broadly from distinct metastatic lesions having unique antigenic repertoires. Extensive genetic evidence from human disease that has been recapitulated in various mouse knock-out studies point to STING as an innate immune sensor that is critical to the development of adaptive cellular immunity. STING is ubiquitously expressed, indicating that it may be difficult to achieve a therapeutic index with systemically delivered specific agonists at a level that is sufficient to activate this pathway in the TME. In contrast, inhibition of TREX-1 preferentially localizes activation of STING to the TME, leading to induction of tumor-specific immunity.
Tempest is currently moving the program through lead optimization.
